Soil Management Guide
Greenhouse Gases in Agriculture
- What is climate change?
- What are the agricultural contributions to climate change?
- What are "Carbon Sinks"?
- Land management to reduce greenhouse gas emissions
- Soil management to reduce greenhouse gas emissions
- Nutrient management to reduce greenhouse gas emissions
- Composting manure to reduce greenhouse gas emissions
What is climate change?
The term “climate change” is commonly used interchangeably with “global warming” and “the greenhouse effect”, but is a more descriptive term. Climate change refers to the buildup of man-made gases in the atmosphere that trap the sun’s heat, causing changes in weather patterns on a global scale. The effects include changes in rainfall patterns, sea level rise, potential droughts, habitat loss and heat stress. (National Safety Council, Environmental Health Centre).
Predicted changes for agriculture in Manitoba due to climate change include (Province of Manitoba, Climate and Green Initiatives):
- More frost-free days would yield a longer growing season, lessen cold stress and reduce winterkill and open up opportunities for new crops. On the other hand, crops could be exposed to more damaging winter thaws, while warmer winter temperatures could decrease the amount of protective snow cover.
- Drought and flooding caused by climate change could increase soil erosion due to wind and water. Loss of protective snow cover would increase the exposure of soils to wind erosion during the winter, while more frequent freeze-thaw cycles could also increase soil erosion.
- Warmer temperatures could lead to increased crop damage from heat stress, as well as an improved breeding environment for a variety of weeds, insects and pests. Droughts, floods and storms could affect the reliability of water for irrigation.
- There would be an increased likelihood of severe drought and increased aridity in semiarid zones of Manitoba.
- Drought, heat waves and the increased frequency of extreme weather events (such as hurricanes, blizzards and ice storms) would affect livestock operations.
The most significant anthropogenic greenhouse gases (GHGs) are carbon dioxide (CO2), methane (CH4) and nitrous oxide (N2O). Although these gases are found naturally in the atmosphere, it is their accelerated increase in concentration due to human activities, most notably burning fossil fuels, that is the concern. Carbon dioxide is the most common GHG but it is not the most potent: CH4 and N2O have 23 and 296 times the global warming potential of CO2, respectively (IPCC, 2001).
What are the agricultural contributions to climate change?
Farming activities in Manitoba, excluding the burning of fossil fuels for heating homes and operating machinery, accounted for about 30% of Manitoba’s total GHG emissions in 2005 (Figure 11.1). This is second only to the GHG emissions arising from the transportation sector. From 1990 to 2005, agriculture-related emissions increased by 36%.
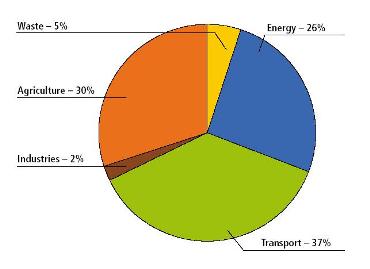 |
Figure 11.1 2005 greenhouse gas emissions in Manitoba by sector (Environment Canada, 2007) |
Agriculture produces CO2, CH4 and N2O. While CO2 is the primary gas emitted by most other industries, the primary greenhouse gases emitted by agriculture are CH4 and N2O. Table 11.1 provides a breakdown of GHGs emitted by agriculture and their sources and causes. It includes home heating and farm machinery as sources of CO2 emissions from fossil fuel burning, although when governments quantify the contributions from agriculture, the gases from these processes are often considered separately. It does not include CO2 produced in the manufacture of nitrogen (N) fertilizers, which is a significant source of GHG.
On-farm CO2 comes from burning fossil fuels to heat homes and run farm machinery, decomposition of organic matter from intensive tillage operations and summerfallow, and crop residue burning.
On-farm CH4 comes from digestive process of ruminant livestock (cattle, sheep and goat burps), anaerobic (without oxygen) decomposition of organic matter in wet soils, riparian areas, wetlands and manure storages.
On-farm N2O comes from nitrification in soil (when ammonium is converted to nitrate in soil), denitrification in soil (anaerobic respiration in soil due to wet soil conditions or high microbial activity where both carbon and nitrate are present) and in the manure storage.
Table 11.1 Greenhouse gases and their global warming potential, agricultural sources and causes. (Adapted from the Climate Change Connection, 2007.)
| Greenhouse Gas | Global Warming Potential1 | Agricultural Sources | Causes |
| Carbon dioxide (CO2) | 1:1 (CO2 equivalent) |
|
|
| Methane (CH4) | 23:1 (23 times more potent than CO2) |
|
|
| Nitrous oxide (N2O) | 296:1 (296 times more potent than CO2) |
|
|
| 1 Global warming potentials (GWPs) are used to compare the abilities of different greenhouse gases to trap heat in the atmosphere. These estimates are from the Third Assessment Report of the intergovernmental Panel on Climate Change (IPCC, 2001). | |||
Of Manitoba's agricultural emissions in 2005 (Figure 11.2), it is estimated that 43% came from agricultural soils (mostly N20), 41% from enteric fermentation (CH4) and 16% from manure management (CH4 and N20). These estimates do not include GHG emissions from fertilizer production.
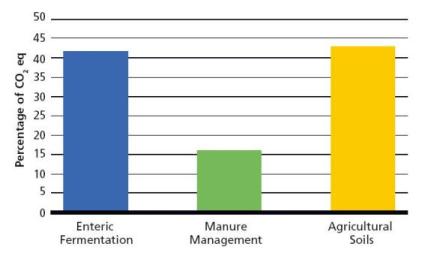 |
Source: National Inventory Report (Environment Canada, 2007) Figure 11.2 Relative proportions of greenhouse gases produced by agriculture (excluding fossil fuel burning for home heating and farm machinery) |
What are "Carbon Sinks"?
Farmers are in the fortunate position of being able to reduce greenhouse gas emissions by increasing their carbon sinks. Carbon sinks are processes that remove greenhouse gases from the atmosphere and store them long-term in another form. On farms, CO2 can be stored as carbon in perennial vegetation (such shelterbelts and woodlots) and in soil as organic matter. Many of the farming practices that reduce greenhouse gases also improve soil quality and productivity, protect water quality and promote profitability.
Land management to reduce greenhouse gas emissions
Marginal lands (agriculture capability Classes 4, 5 and 6) do not have the yield potential of higher class agricultural lands (see agriculture capability explanation in Chapter 2 (Table 2.2)). Some Class 6 and 7 lands have such severe limitations that they are either not profitable or not suited to agriculture. By planting unproductive marginal and often fragile lands to perennial cover, farmers can improve profit margins, create a carbon sink and provide natural habitat (Soil Conservation Council of Canada, 2003).
Agroforestry is a land management approach that combines the production of trees with other crops and/or livestock. Trees, like growing crops, remove CO2 from the air, storing it as carbon in trunks, branches, leaves and roots. By blending agriculture and forestry, particularly on marginal lands, agroforestry can optimize economic and environmental benefits.
Although wetlands are a source of CH4 and N20, all of their advantages should be considered when assessing their ecological value on the farm. Wetlands can remove CO2 from the atmosphere, help to clean water, provide wildlife habitat and reduce downstream flooding. Drained areas of the field that remain less productive due to excess moisture for significant portions of the year (agriculture capability Classes 5W, 6W and 7W) may provide more ecological value if they were restored back to wetlands.
When soils become saturated, soil microbes use nitrate-N to respire instead of oxygen through a process called denitrification. This process results in a loss of N fertilizer to the air as N2O and N2 gases. Improving the drainage on lands with mild to moderate wetness limitations (agriculture capability Classes 2W, 3W and 4W) can reduce greenhouse gas emissions by decreasing denitrification and increasing plant uptake of CO2 by healthier more vigorous crops. Unfortunately, drainage is not without some risk to water quality. Tile drainage and surface drains must be managed in a way that reduces the risk of nutrient transfer to surface water. As well, improving drainage on some lands may increase the oxidation of organic matter and release of CO2.
Soil management to reduce greenhouse gas emissions
Agricultural soil is dynamic biological system that both stores and releases greenhouse gases. Whether or not the soil acts as a net source of CO2 or a net sink for CO2 can be influenced by soil management. By increasing soil organic matter levels – a process called carbon sequestration – the farmer can decrease CO2 emissions and increases the soil carbon sink.
Soil organic matter levels can be increased by producing healthier crops and reducing tillage operations. Healthy crops not only produce more harvestable material for the farmer but they also decrease greenhouse gases by trapping more carbon in their roots, some of which will be converted to more stable soil organic matter. Conservation tillage systems increase soil organic matter levels by decreasing the amount of organic matter that is oxidized and released to the atmosphere as CO2. In conservation tillage, crops are planted into the previous year’s stubble with minimum or no tillage. In addition to increasing soil organic matter levels, this practice also reduces fossil fuel consumption and reduces the risk of soil erosion by wind, water and tillage. It is estimated that conservation tillage, along with reduced use of summerfallow, can store from 0.3 to 0.5 tonnes of carbon per hectare per year in the soil, depending on weather and moisture conditions (Soil Conservation Council of Canada, 2006).
The use of perennial forages in crop rotations reduces GHG emissions by increasing carbon storage (sequestration) in agricultural soils. For example, perennial forages can sequester 2 to 3 more tonnes of CO2 per hectare per year than annual crops (Grant et al. 2004). Alfalfa can also fix its own atmospheric N, thereby eliminating the need for commercial fertilizer applications in the years following establishment. This is an additional GHG reduction benefit because both the production and application of N fertilizer involve the burning of fossil fuels.
Nutrient management to reduce greenhouse gas emissions
The use of N fertilizers, whether commercial inorganic fertilizer or manure, increases GHG emissions from soil. When ammonium is added to soil, it is converted to nitrate by soil microorganisms through a process called nitrification. This process requires oxygen and releases small amounts of N2O. In anaerobic soils, nitrate is converted to N gases through a process called denitrification. Denitrification occurs in the absence of oxygen, requires both carbon and nitrate and gives off N2 and N2O.
Good nutrient management practices help to reduce GHG emissions. Fertilizer type, application rate, timing and placement have been shown to influence the amount of N2O released to the atmosphere from some soils in some years (Burton et al. 2007). Improved fertilizer efficiency represents an economic savings for the producer and will reduce the amount of excess N fertilizer that can be lost to the atmosphere or to surface or groundwater. Any reduction in commercial N fertilizer use has the added benefit of reducing the greenhouse gases emitted during its manufacture.
The first step towards improving fertilizer efficiency is determining how much fertilizer N the crop requires. Nitrogen application rates should take into account how much available N is already in the soil and any additional N requirements should target realistic crop yields. This is achieved through annual soil testing for residual soil nitrate levels. Targeted N application rates will minimize the amount of nitrate that is remaining in the soil after the crop has been harvested. This excess nitrate is at increased risk of being lost to the atmosphere as N2O the following spring when soils are saturated during snowmelt. Additional benefits of targeted N application rates include optimal crop response, reduced crop lodging, reduced risk of nitrate leaching to groundwater and decreased fertilizer costs.
Manure is an excellent source of nutrients for crop production and can replace the requirement for commercial fertilizer. Like fertilizer, manure should be applied at rates that meet crop nutrient requirements. Unlike commercial fertilizer, however, manure is a heterogeneous mix of nutrients, organic matter and water. The only way to know the nutrient concentration of manure is through laboratory analysis of a representative, composite manure sample. Similar to commercial fertilizer, spring applications of manure are ideal but are not always practical. Winter applications of manure should be eliminated to prevent runoff, leaching and volatilization of ammonia. To ensure the target application rate of commercial fertilizer or manure is applied, application equipment must be calibrated.
The N in manure and ammonium-based fertilizers is at increased risk of being lost to the atmosphere as ammonia (NH3) gas. These losses can result in indirect GHG emissions when the ammonia is re-deposited on land elsewhere and lost as N2O. Injection or immediate incorporation of manure and ammonium-based fertilizers can reduce or eliminate volatilization of NH3. This not only reduces indirect GHG emissions, but it can represent a significant savings for the producer in N fertilizer.
Ideally, fertilizers should be applied as close as possible to the time that plants need them. Applications of fertilizers in the spring after snowmelt reduce the risk of losses to the environment during spring snowmelt. During the snowmelt period, denitrification rates can be high if nitrate and carbon are present in the soil because the soil is often also saturated. Late fall applications of ammonia-based N, when soils are cool, are also acceptable as much of the N is not converted to nitrate until the soils warm again the following spring after snowmelt. One of the most efficient methods of fertilizing annual crops is banding the fertilizer at seeding. If banding is not possible, then incorporation as soon as possible after application will reduce the risk of losses to the environment. Some long-season, wide row crops such as corn and potatoes permit in-season application of N, which may be the most efficient time to apply N fertilizer.
Slow release N fertilizers supply N more slowly over the growing season when the crop can use it and reduce the risk of N loss to the environment. Slow release fertilizers are more expensive, however, so economics may limit their use. Urease and nitrification inhibitors improve the efficiency of N uptake and are more affordable than slow-release fertilizers. Urease inhibitors prevent volatilization of surface-applied urea and indirect GHG emissions. Nitrification inhibitors slow the conversion of ammonium-N to nitrate-N and have been shown to reduce N2O emissions in some soils.
Inclusion of leguminous cover crops or green manure crops in crop rotations could also decrease GHG emissions. The more gradual release of the N from these crops over the subsequent growing season may result in less N in the soil at any one time that is susceptible to loss as N2O following precipitation events. As well, crediting the N from these crops reduces the requirement for commercial N fertilizer for the next crop. The reduction in N fertilizer use means that less greenhouse gases are emitted from N fertilizer manufacture.
Composting manure to reduce greenhouse gas emissions
Composting manure is the controlled, accelerated decomposition of manure into a more stable organic form. Composting solid manure reduces the volume for land application by up to 50% thereby decreasing application costs for the producer and possibly reducing the use of fossil fuels.
The addition of compost to soil improves soil quality. Compost improves soil organic matter levels, decreases bulk density and increases fertility, aeration and water holding capacity. The use of compost may reduce the need for commercial fertilizers.
The overall benefits of composting manure for greenhouse gas reduction are promising although reduction estimates are variable and depend on the method of composting and type of manure. More research in this area is required before these benefits can be quantified.
